While screening programs for several of the commonest cancers are now well established – like breast or colon cancer for example – lung cancer screening has yet to reach anywhere near the same proportion of at-risk patients. A recent report by the American Lung Association concluded that in 2021 less than 6% of eligible Americans were screened for lung cancer [1]. This low screening rate is almost certainly a contributor to the fact that lung cancer stubbornly remains the world’s leading cause of cancer death. And the numbers are high. Lung cancer is second only to breast cancer in terms of the number of new cases each year.
The arguments against the effectiveness of lung cancer screening go back more than 30 years, among them the belief that the high number of false positives generated would lead to overdiagnosis, resulting in unnecessary patient anxiety and invasive procedures; that annual screening would exceed a patient’s recommended cumulative radiation dose; and that it would stigmatize smokers – one of the highest risk target groups.
“The first thing we need to do to improve lung cancer screening rates is to encourage greater participation in those screening programs that do exist, through better education and outreach, increased clinician referrals, improved accessibility, and wider reimbursement. For example, in partnership with Roswell Park Comprehensive Cancer Center in Buffalo, NY, Philips and Roswell Park are expanding access to early lung cancer screening via Project EDDY (Early Detection Driven to You), bringing mobile low dose CT lung cancer screening to at-risk populations of medically underserved and racially diverse populations in rural and metro areas of New York.” – Dr. Ilya Gipp, MD, PhD, Chief Medical Officer Oncology, Precision Diagnosis at Philips
“The second thing is to improve our eligibility criteria, which at present are largely based on just two simple parameters – age and smoking history. But it isn’t just high ‘pack-year’ smokers who are at increased risk of getting lung cancer. All sorts of professions, from firefighters and asbestos workers to military personnel and welders are, or have been exposed, to lung cancer causing carcinogens. As clinicians, we surely owe them all the same duty of care.” – Dr. Ilya Gipp
Detect early, cure fast
The key to improving the chances of curing virtually any form of cancer starts with early detection and timely treatment. Is early detection and timely treatment of lung cancer winnable? The answer is most certainly yes. But to do so, there is a need to re-evaluate every step in the risk assessment, screening, diagnosis, treatment, and follow-up process, taking an integrated end-to-end approach so that improvements in one part of the care cycle don’t create bottlenecks in its other parts.
While a chest CT (computed tomography) scan remains the standard imaging modality for lung cancer screening, detection, and diagnosis, what’s needed to reduce the number of false positives and extend participation to risk groups other than smokers is better risk assessment tools. The availability of simple risk assessment tools for colon cancer, in the form of easy-to-use stool tests, has revolutionized eligibility for colonoscopy, allowing interventionists to detect and remove tumors even before they become cancerous. The good news is new blood or sputum-based liquid biopsies could now do the same for assessing an individual’s lung cancer risk and eligibility for CT screening, as well as helping reduce the number of false-positives, improve participation rates, and increase lung cancer detection rates [2]. Promising tests are already going through approval in the U.S.
Never waste an image
While a low-dose CT will almost certainly remain the primary screening and diagnostic tool for lung cancer, it should not be forgotten that chest CTs are performed for a wide range of other common pulmonary disorders such as pneumonia, COPD, and pulmonary embolism; cardiovascular disorders such as coronary artery disease, aortic aneurysm, and pulmonary hypertension; and many other chest pathologies. Many of these scans are capable of revealing incidental findings of potentially meaningful lung nodules not only in smokers but also in other patient groups. In reality, however, many incidental findings are missed and of those that are recorded as few as one in four are properly followed up. Yet up to a quarter of pulmonary nodules detected incidentally and correctly followed up have proved to be malignant [3].
What if computers could help read every chest CT image or radiology report for potential lung nodules, drawing physicians’ attention to the need to refer the patient for further investigation and follow-up. A Philips-sponsored screening program for miners in Australia initially designed to detect black lung disease (pneumoconiosis) is now also actively analyzing CT images for both lung cancer and cardiovascular disease. In time, it may be possible to train artificial intelligence (AI) to detect early-stage lung cancer in a simple non-CT chest X-ray. In developing countries, where the cost of a CT scan is prohibitive or inaccessible for large parts of the population, that could be a real game-changer.
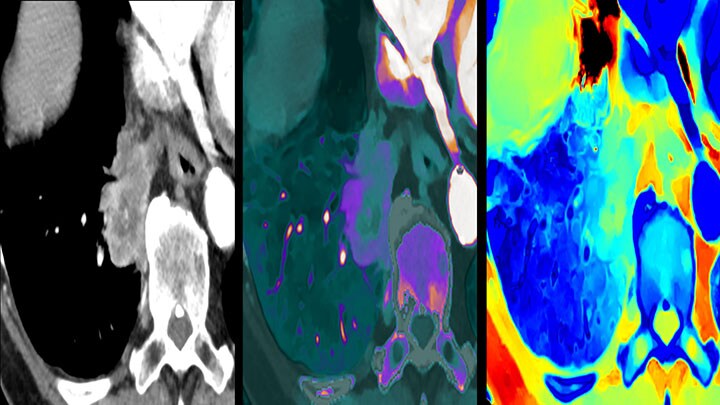
It’s not just the image, it’s the crucial data within the image
Low-dose CT is getting very good at identifying lung nodules as small as a few millimeters in size, well before they are likely to do significant damage. If novel risk assessment tools are better able to identify patients who are likely to have lung nodules and the existence of one or more nodules in their lungs is subsequently revealed by screening, the next big question is how do we identify which nodules need to be referred for immediate biopsy – the gold-standard for determining malignancy – and which ones can be followed up at lower priority? Without tools to answer these questions we may well be back to the false-positive scenario. Currently, around 50% of lung nodule biopsies come back negative.
“One of the latest developments in this field is undoubtedly spectral-detector CT – a technology that allows us to quantify a nodule’s level of iodine contrast media uptake and hence its probability of malignancy. While not a definitive indicator of cancer, it can provide valuable information to decide whether a tumor simply needs watching, in which case the patient can be enrolled into a routine follow-up program, or whether it needs to be biopsied immediately. If the spectral information is effortlessly collected by default with every scan, as is the case for Philips’ Spectral CT 7500 scanner, radiologists can always refer to multi-energy spectral data in any exam to take a closer look.”- Dr. Ilya Gipp
Multi-faceted information – integrated imaging, pathology, genomics, and proteomics containing vast amounts of biomarker information – is key to making the right treatment decisions for lung cancer patients. A group of researchers in the U.S. have even discovered a correlation between the twistedness (quantitative vessel tortuosity) of tumor vascularity revealed by routine CT scans as a predictor of patient response to immune checkpoint inhibitor (ICI) immunotherapy [4]. CT images, especially those with captured spectral data, are also helping us better understand a cancerous lung tumor’s response to treatment. For example, spectral CT not only allows us to see the overall mass of a tumor, it also allows us to differentiate between necrotic and active volumes within the mass. For chemotherapy or radiation therapy it can provide timely information to help determine if we are on the right path or whether we need to find an alternative treatment pathway to get the patient cancer-free.
The CT-like images captured by cone-beam CT (CBCT) are being used for real-time guidance on systems such as Philips’ Image Guided Therapy System – Azurion – to help guide needle and catheter-based biopsies and make minimally-invasive procedures such as lung tumor ablation safer and easier.
On the horizon
While CT remains at the heart of every step in the lung cancer diagnosis and treatment pathway, enabling clinicians to identify, treat, and follow-up the growing number of patients that have developed the disease, the ultimate aim is to identify lung cancer at Stage 0 or maybe Stage 1A or 1B, where it can be surgically removed or destroyed by relatively straightforward minimally-invasive procedures.
We are not there yet, but thanks to major ongoing collaborations such as I-ELCAP (The International Early Lung Cancer Action Program) major progress is already being made. I-ELCAP research has already indicated that annual CT screening has the potential to diagnose 80% of lung cancers at Stage 1, with 80% to 90% of those lung cancers being curable. What’s more, they have concluded that the associated screening costs compare favorably with those for breast, cervical, and colon cancer [5].
The early detection and treatment of lung cancer is definitely winnable, and it may not be too far away.
[1] American Lung Association. State of Lung Cancer 2022 Report. https://www.lung.org/getmedia/647c433b-4cbc-4be6-9312-2fa9a449d489/solc-2022-print-report
[2] International Association for the Study of Lung Cancer. Liquid biopsy for early lung cancer detection and cancer interception https://www.ilcn.org/liquid-biopsy-for-early-lung-cancer-detection-and-cancer-interception/
[3] Tanner NT, Aggarwal J, Gould MK, Kearney P, et al. Management of Pulmonary Nodules by Community Pulmonologists: A Multicenter Observational Study. Chest. 2015 Dec;148(6):1405-1414. doi: 10.1378/chest.15-0630. PMID: 26087071; PMCID: PMC4665735. https://pubmed.ncbi.nlm.nih.gov/26087071/
[4] Mehdi Alilou et al. ,A tumor vasculature–based imaging biomarker for predicting response and survival in patients with lung cancer treated with checkpoint inhibitors. Sci. Adv.8,eabq4609(2022).DOI:10.1126/sciadv.abq4609 https://www.science.org/doi/10.1126/sciadv.abq4609
[5] https://www.ielcap.org/home/ielcap/
Share on social media
Topics
Contact

Anna Hogrebe
Philips Global Press Office Tel: +1 416 270 67 57
You are about to visit a Philips global content page
Continue