Aug 11, 2020
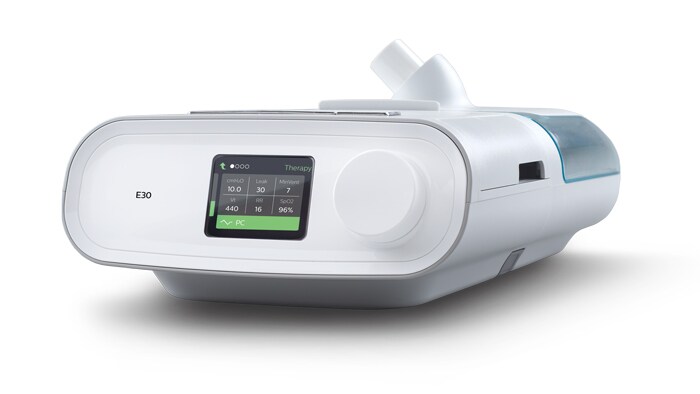
Philips introduces new Philips Respironics E30 ventilator in Kenya in wake of COVID-19 spike
Nairobi, Kenya: Philips today announced that it has received local emergency use authorisations[1] to release its new Philips Respironics E30 ventilator in Kenya and assist in filling the critical hospital ventilation shortage. The Philips Respironics E30 Ventilator has been verified and approved by the Pharmacy and Poisons Board which is the body mandated to verify and approve medical devices in Kenya. As of June 2020, the Ministry of Health indicated that Kenya had only 189 ventilators countrywide to treat critical care COVID 19 patients. It is in response to this, and similar needs globally, that Philips quickly scaled production of its new Philips Respironics E30 ventilator as a readily available ventilation alternative during the COVID-19 crisis in situations where full-featured, critical care ventilators are not available.
Designed for mass production by a team deeply experienced in respiratory care, Philips has upscaled production of the device since mid-April 2020, producing 15,000 units per week to free-up intensive care and critical care beds, while allowing healthcare workers with a wide range of skill sets to treat and monitor patients in clinical and field-hospital settings. Studies continue to show that there is a strong association between people who are critically ill or dying and those with certain chronic conditions. The three most commonly associated chronic conditions are hypertension, diabetes and obesity, as well as lung diseases such as asthma and chronic obstructive airways diseases. This supports the crucial need for local healthcare to scale up access to COVID-19 related interventions, as non-communicable diseases (NCD’S) such as cancers, diabetes and others account for 27 per cent of the total deaths and over 50 per cent of total hospital admissions in Kenya – putting the Kenyan population at unprecedented risk.
The E30 ventilator features include:
As COVID-19 continues to spread in Kenya, healthcare providers are working diligently to treat soaring numbers of patients at a time when there are too few ventilators to provide care
Prof. Wangari Siika
Associate Professor in Anaesthesia and Intensive care.
The E30 ventilator is fit for purpose, specifically made for COVID-19 management. It is not a full-featured, critical care ICU ventilator, but has been developed keeping the needs of healthcare workers and COVID-19 patients in mind while also complying with medical device quality standards” stated Dr. Muthoni Ntonjira, Country Manager, Philips Kenya. “Our hope is that this ventilator will help to free-up ICU ventilators for use in treating the most severe patients and support the efforts being made by our Government and our clinical workforce.” For further information, please contact: Radhika Choksey Head of Marketing – Philips Africa Tel: +31 62525 9000
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.